Wednesday, February 8, 2012
New Views on Opioid Equivalency

By guest author, Dmitry M. Arbuck, MD
Opioid equivalency — how the various opioid analgesics compare with each other in potency and effect — is of more than theoretical importance, and currently there is no universally accepted standard regarding this subject [10,19,21,22]. This is largely due to the diversity of patient sensitivity to the specific opioid molecules in question; that is, the potency of each opioid may depend not only on its molecular structure, but also on its activity and metabolism as well as opioid-receptor composition in each patient.
Nevertheless, we need some general guidance on how to view and compare opioid potency, which becomes of special importance when switching a patient from one opioid analgesic to another [11,12]. Accepted clinical practice suggests starting any new opioid at a dose of 1/2 to 1/3 of the dose of the first opioid. This facilitates a significantly diminished risk of opioid-induced respiratory depression due to incomplete cross-tolerance between opioids. That is, two different opioids at exactly the same dose may have much different effects, and potentially harmful so, in an individual patient — even if the patient has been already taking one of the opioids for some time. Overall, the subject of opioid equivalency is complex on several levels.
Analgesic Equivalency – Equianalgesia
The most common perspective of equivalency relates to analgesic equivalency, or equianalgesia [17]. Opioids also may be compared in their rate of induction of respiratory depression, and the likelihood of inducing constipation, GI spasm, lower leg edema, sweating, psychosis, and a myriad of other effects. Nevertheless, modern equivalency charts relate primarily to the analgesic potency of opioids [14,16].
An assumption is that respiratory depression directly relates to the analgesic, pain-relieving property. This may or may not be true and requires additional research to shed light on the relationship between opioid-induced analgesia and respiratory depression. Comparisons of opioids to morphine may be found in the literature, but direct, head-to-head comparisons between the other opioids are rare [6,15].
One view of equianalgesic comparisons of opioids is reflected in the description of opioid tolerance in prescribing information for rapid-onset fentanyl-based medications approved for breakthrough cancer pain. This states that an opioid-tolerant patient is one who takes either 25 mcg/hour of transdermal fentanyl, 60 mg per day of oral morphine, 30 mg/day of oxycodone, or 8 mg/day of hydromorphone [1,2,3,4].
By these assumptions, fentanyl is 100 times more potent than morphine, oxycodone is twice as potent as morphine, and hydromorphone is 7.5 times more potent than morphine. In contrast, however, many practitioners find that the potency of morphine, oxycodone, as well as hydrocodone, are equal or closely similar in value, and the potency of hydromorphone also seems to be overestimated in the FDA-approved product information.
“Liking” Makes a Difference
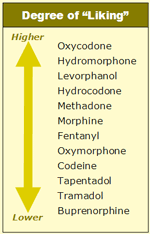
From a practical standpoint, one of the most important comparisons between opioids is based not just on the extent of analgesia and respiratory depression, but also on the tendency for inducing a “liking” phenomenon. This is a troublesome aspect because it is a cornerstone of the abuse and addictive potential of opioids [23]. From my own clinical experience in treating patients, the degree of liking produced by the various opioid medications can be ranked according to the table at left.
As noted above, head-to-head studies comparing multiple opioid agonists are rare, and the traditional “hot plate” test of analgesic effects used in animal studies is not entirely translated into humans because the liking component — which is so important in subjectively estimating opioid potency — cannot be reliably evaluated. Nevertheless, this test consists of placing a laboratory animal on a hot plate and registering the temperature level tolerated by the animal after being administered a certain amount of opioid. The higher the temperature the animal can tolerate, the more potent the opioid. Tail flicking is another sign used to evaluate pain control in laboratory animals. Obviously, this sort of testing applies only to an acute nociceptive pain model; the design of a reliable test in chronic pain is much more complex, since it relies on subjective self-reports and lacks truly objective measurements.
Lipophilicity Affects Potency
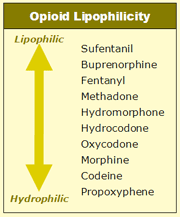
Lipophilicity — which means “fat liking” — refers to a drug’s ability to dissolve in fatty substances and more easily cross into cells and brain tissues; as opposed to hydrophilic (“water-loving”) drugs, which more readily interact with water-based substances and less readily cross cell membranes. A general rule is that the more lipophilic an opioid is the more effective it becomes as an analgesic. The table at right estimates a ranking of opioids on the basis of their lipophilic versus hydrophilic properties.
This ranking is somewhat closely aligned with the order of analgesic potency (see figure below), with more potent analgesics being more lipophilic. Although, this correlation is not exact, which might be due to differences in the level of liking that certain opioids produce.
Opioid-NSAID Comparisons
The only group of medications, in addition to opioids, that are helpful in the acute nociceptive-pain model would be the nonsteroidal anti-inflammatory drugs; therefore, comparisons of opioids and NSAIDS are possible (7). In some cases, NSAIDS may provide comparable or greater analgesia than select opioids, depending on dose, and might be a preferred option for certain patients; NSAIDs lack a “liking” property.
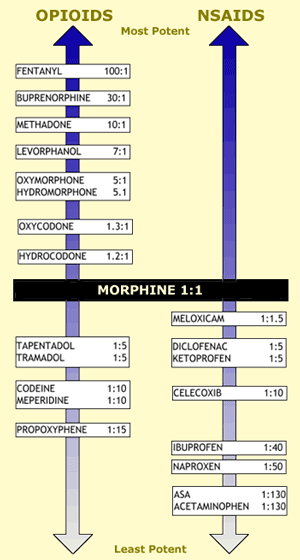
The gold standard for comparison of all pain-relieving medications is morphine, which serves as a reference point for analgesic potency [5,8,9,13]. While more intense research is necessary to evaluate the relative potency of analgesics in a more comprehensive way, the figure at left suggests potency ratios of other analgesics compared with morphine for commonly prescribed agents.
Data in this figure have not been scientifically validated and the presentation is based on clinical experience, which may not coincide with the experiences or opinions of other practitioners. A unique feature is that the figure permits a comparison of various NSAIDS with opioids in terms of relative analgesic potency.
It is important to remember that numeric measures of potency are notoriously unreliable and the ratios should be considered only as general approximations with possible deviations up or down. For example, the fentanyl:morphine ratio could range 70-100:1; buprenorphine 20-40:1; oxymorphone 3-7:1; and so forth. Additionally, lipophilicity plays a role in opioid potency, and “liking” may have a subjective influence on how individual patients respond to particular doses.
An especially important consideration is that methadone has a sliding scale of potency, depending on the number of milligrams administered (due to inhibition of its own metabolism by cytochrome P450 enzyme 3A4) [18,20].
In doses below 10 mg, methadone is almost as potent as morphine in a 1:1 or 1:2 ratio, but methadone doses of about 30 mg are roughly 3 times more potent than morphine (3:1); 70 mg methadone is 7 times more potent (7:1); and, finally, at above 100 mg of methadone potency goes to 10:1 in relation to morphine (table at right). Of course, those ratios should be taken only as general reference points rather than as an absolute dosing guide, since patient physical health (eg, liver function) and potentially interacting concurrent medications could alter methadone metabolism, potency, and adverse effects [see reference 24 for more detailed information].
Multiple Options Worth Considering
It is also important to acknowledge that certain other types of medications — such as antidepressants, anticonvulsants, capsaicin, lidocaine, and others — can produce robust pain relief for certain conditions. Some of these medications are more effective in the chronic pain model than for acute pain; however, ranking them in order of analgesic potency is even more difficult than with opioids due to a wider variation in individual patient responses.
Clinical practices that dictate giving opioids for all types of pain are imprudent; however, this has unfortunately become mainstream in some settings. Dentists frequently give hydrocodone/acetaminophen analgesics to even three year old children. Emergency room physicians commonly provide such opioids to young patients with relatively minor injuries. And, this is especially the case in patients with cancer, when, for some reason, only opioids are considered as providing appropriate analgesia; overlooking NSAIDS or acetaminophen, which are often effective and beneficial.
However, it is important to remember that NSAIDs and acetaminophen also come with their own risks of serious adverse effects, such as liver and kidney problems, or GI ulcerations and bleeding. Still, their use along with opioids or as an alternative in some cases should be revisited and reconsidered by prescribers.

About the Author: Dmitry M. Arbuck, MD, is a psychiatrist specializing in pain management. In 2000 he started Meridian Health Group, a pain management practice that grew into a multidisciplinary pain management facility treating all types of chronic pain. He also has an appointment as a volunteer Assistant Professor of Psychiatry and Medicine at Indiana University School of Medicine and is the former president of the Russian American Medical Association. Dr. Arbuck has served on the speakers’ bureaus of AstraZeneca, Cephalon, Forest Laboratories, King Pharmaceuticals, Metagenics, Pfizer, Reckitt Benckiser, Sanofi, and many other companies. For more information, you can e-mail Dr. Arbuck at [email protected], phone him at 317-814-1000, or visit his clinic website at: http://www.meridianhealthgroup.com.
Proviso: Facts, advice, and opinions expressed above are those of the guest author. Pain Treatment Topics — including our associates, affiliates, and supporters — do not endorse, recommend, or promote the use of any specific medications. Any drugs mentioned by name are strictly for informational purposes and brands are registered trade marks of their respective manufacturers.
REFERENCES:
1. Fentora Prescribing Information, July 2011.
2. Onsolis Prescribing Information.
3. Abstral Prescribing Information.
4. Actiq Prescribing Information.
5. Kawano C, Hirayama T, Kuroyama M, et al. Dose conversion in opioid rotation from continuous intravenous infusion of morphine hydrochloride injection to fentanyl patch in the management of cancer pain. Yakugaku Zasshi. 2011 Mar;131(3):463-467.
6. Kampe S, Wolter K, Warm M, et al. Clinical equivalence of controlled-release oxycodone 20 mg and controlled-release tramadol 200 mg after surgery for breast cancer. Pharmacology. 2009;84(5):276-281.
7. Friday JH, Kanegaye JT, McCaslin I, et al. Ibuprofen provides analgesia equivalent to acetaminophen-codeine in the treatment of acute pain in children with extremity injuries: a randomized clinical trial. Acad Emerg Med. 2009;16(8):711-716.
8. Dunbar PJ, Chapman CR, Buckley FP, Gavrin JR. Clinical analgesic equivalence for morphine and hydromorphone with prolonged PCA. Pain. 1996;68(2-3):265-270.
9. Eddy NB, Lee LE Jr. The analgesic equivalence to morphine and relative side action liability of oxymorphone (14-hydroxydihydro morphinone). J Pharmacol Exp Ther. 1959;125(2):116-121.
10. Shaheen PE, Walsh D, Lasheen W, et al. Opioid equianalgesic tables: are they all equally dangerous? J Pain Symptom Manage. 2009;38(3):409-417.
11. Bond BS. Equianalgesia: applying evidence-based practice guidelines. Clin J Oncol Nurs. 2008;12(3):527-529.
12. Berdine HJ, Nesbit SA. Equianalgesic dosing of opioids. J Pain Palliat Care Pharmacother. 2006;20(4):79-84.
13. Ripamonti C, Groff L, Brunelli C, et al. Switching from morphine to oral methadone in treating cancer pain: what is the equianalgesic dose ratio? J Clin Oncol. 1998 Oct;16(10):3216-3221.
14. Mercadante S, Caraceni A. Conversion ratios for opioid switching in the treatment of cancer pain: a systematic review. Palliat Med. 2011;25(5):504-515.
15. Etropolski M, Kelly K, Okamoto A, Rauschkolb C. Comparable efficacy and superior gastrointestinal tolerability (nausea, vomiting, constipation) of tapentadol compared with oxycodone hydrochloride. Adv Ther. 2011;28(5):401-417.
16. McNicol E. Opioid equianalgesic conversions. J Pain Palliat Care Pharmacother. 2009;23(4):458.
17. Knotkova H, Fine PG, Portenoy RK. Opioid rotation: the science and the limitations of the equianalgesic dose table. J Pain Symptom Manage. 2009;38(3):426-439.
18. Walker PW, Palla S, Pei BL, et al. Switching from methadone to a different opioid: what is the equianalgesic dose ratio? J Palliat Med. 2008;11(8):1103-1108.
19. O’Bryant CL, Linnebur SA, Yamashita TE, Kutner JS. Inconsistencies in opioid equianalgesic ratios: clinical and research implications. J Pain Palliat Care Pharmacother. 2008;22(4):282-290.
20. Chhabra S, Bull J. Methadone. Am J Hosp Palliat Care. 2008;25(2):146-150.
21. Berdine HJ, Nesbit SA. Equianalgesic dosing of opioids. J Pain Palliat Care Pharmacother. 2006;20(4):79-84.
22. Gordon DB, Stevenson KK, Griffie J, et al. Opioid equianalgesic calculations. J Palliat Med. 1999;2(2):209-218.
23. Vallejo R, Barkin R, Wang V. Pharmacology of opioids in the treatment of chronic pain syndromes. Pain Physician. 2011;14:E343-E360.
24. Toombs J. Oral Methadone Dosing for Chronic Pain: A Practitioner’s Guide. Pain Treatment Topics. Updated March 2008 [PDF available here].
