Thursday, June 10, 2010
Chilling Concept: Chronic Pain as a Brain Disease
There has been ongoing debate over whether chronic noncancer pain is a syndrome or a disease entity unto itself. Compelling research evidence suggests that no matter what brought on the condition — acute injury, precipitating disease, or unknown factors — chronic pain ultimately manifests as a distinct brain disease, which impacts all aspects of the individual’s life and may become irreversible. Appropriate clinical response to this alarming notion could require prompt diagnosis followed by aggressive, multimodal pain management early in the course of disease progression to forestall or ameliorate potentially serious and incapacitating brain damage.
Need for a Paradigm Shift
In an excellent review examining current perceptions among healthcare professionals and the public regarding chronic pain, Tracey and Bushnell [2009] observe that chronic pain has historically been labeled as a syndrome (or group of syndromes or symptoms consistently occurring together). They argue, however, that recent evidence coming largely from neuroimaging research strongly advocates for chronic pain being labeled as a disease. Therefore, they contend, “a paradigm shift in our thinking is needed if we are to better diagnose, manage, and treat chronic pain.”
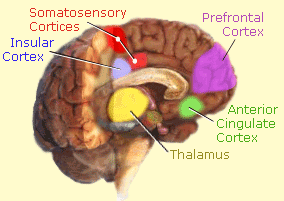
Relatively new, noninvasive neuroimaging and electrophysiological technologies have facilitated unprecedented examinations of how both acute and chronic pain affect signal processing (functionality), metabolic activity, and structural changes in the living human brain [Geha and Apkarian 2005; Tracey 2008]. Various investigations and data analyses from the laboratory of A. Vania Apkarian — at Northwestern University Feinberg School of Medicine, Chicago, IL — have identified 6 key brain structures as being of critical importance in all acute and chronic pain conditions: insular cortex, thalamus, cingulate cortex, somatosensory cortices (S1 and S2), and the prefrontal cortex [Apkarian et al. 2005, see schematic illustration]. In some studies additional structures, primarily within limbic regions and what has been more vaguely termed the “pain matrix,” have been found to play roles; however, a major feature distinguishing chronic pain is significantly dominant involvement of the prefrontal cortex.
The prefrontal cortex, which has a number of components, is a major control center generally responsible for cognitive processing of the pain experience (thinking) and the assignment of emotional meaning as the individual tries to internally manage or cope with daily suffering (including fear and anxiety, along with the pain itself) [Apkarian et al. 2009]. Interconnectivity among the various structures is complex and extensive: the thalamus and the somatosensory cortices are typically associated with sensory aspects of pain processing; whereas the cingulate and insular cortices, plus closely aligned limbic structures and the prefrontal cortex, are associated with pain memory, cognition, and emotional responses to pain [Apkarian et al. 2005, 2009; Grant et al. 2010].
From a clinical perspective, knowledge of the specific brain structures involved in chronic pain may be less important than an understanding of the overarching principles. Apkarian et al. [2009] note that chronic pain is an integrated sensory, emotional, and hedonic (ie, overall sense of well-being) experience. The transition from acute to chronic pain involves a neurobiological reorganization and a transition in the salience of pain “wherein the condition shifts from viewing a painful percept as a sign of external threat into an indication of an internalized disease state.” In essence, chronic pain of any sort is a much different “beast” than being merely acute pain that has continued for a long time.
The Brain Damage of Chronic Pain
To date, various chronic pain conditions have been investigated and a common finding is that at some undetermined point in time there is a degeneration of gray matter volume and density in critical brain regions that may become irreversible. For example, neuroimaging studies using morphometric analyses have shown that the brains of patients with fibromyalgia exhibit 3.3 times greater decreases in gray matter than healthy age-matched controls; in effect, each year that a patient has fibromyalgia is equivalent to 9.5 times greater loss of gray matter than they would expectedly have due to normal aging [Kuchinad et al. 2007]. Similarly, Apkarian and colleagues were the first to show that chronic back pain reduces gray matter (primarily in the prefrontal cortex and thalamus) by 5% to 11% compared with healthy control subjects, which is equivalent to the amount of gray matter volume lost in up to 20 years of normal aging [Apkarian et al. 2004]. Other chronic pain conditions — eg, neuropathies and complex regional pain syndrome (CRPS) [Geha and Apkarian 2005] — have been examined and similar gray matter deficits discovered.
One plausible theory for the gray matter degeneration, proposed by Apkarian and co-investigators [2004], is that the physiological and mental stresses and strains of coping with continuous pain (and concomitant excitotoxic and/or inflammatory mechanisms) result in “overuse atrophy”; essentially, neuronal functioning in affected brain regions becomes exhausted leading to apoptosis (cell death) and shrinkage of gray matter in both volume and density. Interestingly, and seemingly in support of this theory, researchers at McGill University in Montreal, Quebec, found that select limbic structures in the brains of women with vulvodynia (provoked vestibulodynia) of relatively short duration (1 to 9 years) exhibited increased gray matter density, as if there were cellular upregulation in an attempt to modulate pain and attenuate associated symptoms; whereas, older patients with more longstanding vulvodynia demonstrated the typical gray matter degeneration associated with unrelenting chronic pain [Schweinhardt et al. 2008]. However, it cannot be determined from research reports if this same pattern of increase then decrease occurs in other conditions that have been studied. The relative contributions in grey matter shrinkage due to attrition of neurons, axon/dendrite branches, glial cells, blood vessels, and interstitial fluid still needs to be defined. Apkarian et al. [2004] suggest that the atrophy primarily results from a degeneration of interneurons within the affected brain structures.
What Are the Implications?
Taken as a whole, imaging and electrophysiological research studies conducted primarily during the past decade have uncovered 3 trends establishing chronic pain as a brain disease [Tracey 2008; Tracey and Bushnell 2009]:
-
- Chemical Changes: Various studies have shown that persons with chronic pain have decreased concentrations of several chemicals in key brain structures associated with pain: glutamate (an excitatory and facilitative neurotransmitter), dopamine (a monoamine neurotransmitter affecting the sympathetic nervous system and the modulation of emotions, pleasure, and pain in limbic regions), and N-Acetyl aspartate (a marker when deficient of neurodegenerative conditions).
-
- Functional Changes: Research has clearly demonstrated dysfunctional patterns of brain activation in response to various chronic pain conditions compared with healthy controls or subjects with acute pain. These functional changes result in a reduced capacity to inhibit painful stimuli and overactivity in brain areas that make the experience of pain more unpleasant; consequently, the person becomes less able to cope with the pain physically, cognitively, and emotionally.
- Structural Changes: At some point during the course of chronic pain progression gray matter (which is usually dense with nerve cell bodies) in the thalamus, prefrontal cortex, and certain closely aligned areas, becomes reduced in volume and density. It is hypothesized that this may be due to excitotoxicity (continuous nerve stimulation) and/or inflammatory processes resulting in eventual neurodegeneration.
In sum, the brains of persons with chronic pain conditions exhibit premature aging and deterioration affecting normal functionality that should be of great concern to healthcare providers and patients. If current survey data are at least somewhat accurate, chronic pain affects roughly 1 in every 5 adults [Breivik et al. 2006; Tracey 2008] and up to 10% of the population suffers severe chronic pain [Harstall and Ospina 2003], making it a public health threat of epic proportions. However, the percentage of those victims that have developed serious brain “lesions” wrought by the neurobiological changes of chronic pain as a disease process is undetermined.
The sort of changes due to chronic pain described by the research would appear to result in neurobiological dysfunctions that could impact not only a patient’s quality of life but also their cognitive and emotional stability on a day-to-day basis. This raises several questions of concern: Could the changes in brain function and structure contribute to the higher mortality risk observed in patients with chronic pain [previously discussed here]? Could gray matter degeneration account for the cognitive deficits, including “fibro fog,” that plague many patients afflicted with fibromyalgia? Do structural changes, particularly in limbic regions and the prefrontal cortex, contribute to a propensity for developing addiction to analgesic medications? These and other questions need further consideration.
So, What Can Be Done?
A more extensive discussion of potential remedial actions is reserved for a future blogpost in this series; however, a few points are worth quickly noting. The implications from research evidence are that chronic pain of any type or etiology could be considered a serious and in many cases life-threatening brain disease. Clinically, this suggests that there is considerable urgency in the proper diagnosis and aggressive treatment of chronic pain at the earliest possible stages. If improperly treated or undertreated, it seems apparent that the brain disease will progressively worsen, although the timeline may depend on the specific condition, brain areas most affected, and various personal factors such as overall physical status, age, genetics, environment, and still unknown influences. And, although the human brain appears to have a great deal of plasticity or capacity to remodel itself, the extent to which the degenerative changes of chronic pain can be reversed is a vital question needing further research.
Many of the medications used in managing chronic pain modulate brain chemistry but it is unknown if these effects are purely palliative or actually might assist in remodeling brain architecture for the better. At the least, even temporary relief of pain could facilitate patients’ participation in a host of complementary or adjunctive therapies as part of an integrated approach, for example:
-
- In an earlier post [here] we noted that coping skills training appears to facilitate better management of the pain experience by the prefrontal cortex, which also suggests a potential role for hypnosis, guided imagery, and a variety of other stress reduction approaches.
-
- Tai Chi was found to benefit acute pain reduction and psychological health [described here], which might bolster functioning of the prefrontal cortex in chronic pain.
-
- Recent research has demonstrated that Zen meditation facilitates cortical thickening in the cingulate and somatosensory regions that might be of benefit in remodeling brain deficits [Grant et al. 2010].
- Even the words used during practitioner-patient interactions appear to positively or negatively affect activity in the brain’s pain matrix [discussed here] and could influence cognitive processing of pain.
Clearly, further research is needed. Meanwhile, some pain practitioners, such as Forest Tennant, MD, DrPH, have been proactively recommending the aggressive application of both traditional and nontraditional therapies, including the use of nutraceuticals, diet, appropriate mental exercise, and other modalities, in concert with pharmacologic and interventional approaches [Tennant 2007, 2009]. There appear to be many therapeutic possibilities if one accepts the concept of chronic pain as a brain disease and thinks in terms not only of pain relief but of how brain function and structure might be rehabilitated.
REFERENCES:
> Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81-97 [PDF here].
> Apkarian AV, Bushnell C, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463-484 [PDF here].
> Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. Pain. 2004;24(46):10410-10415 [PDF here].
> Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. 2006;10(4):287-333 [PDF here].
> Geha PY, Apkarian. Brain imaging findings in neuropathic pain. Curr Pain Headache Rep. 2005;9:184-188 [PDF here].
> Grant JA, Courtemanche J, Duerden E, et al. Cortical thickness and pain sensitivity in Zen meditators. Emotion. 2010;10(1):43-53 [abstract].
> Harstall C, Ospina M. How prevalent is chronic pain? Pain Clinical Updates. 2003;11:1–4 [PDF here].
> Kuchinad A, Schweinhardt P, Seminowicz DA, et al. Accelerated Brain Gray Matter Loss in Fibromyalgia Patients: Premature Aging of the Brain? J Neuroscience. 2007;27(15):4004–4007 [PDF here].
> Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140(3):411-419 [abstract].
> Tennant F. Intractable Pain Patient’s Handbook for Survival. Pain Treatment Topics. 2007 [PDF here].
> Tennant F. Brain atrophy with chronic pain. Prac Pain Manage. 2009;9(2):12-14 [abstract].
> Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: Is chronic pain a disease. J Pain. 2009;10(11):1113-1120 [abstract].
> Tracey I. Imaging pain. Br J Anaesthesia. 2008;101(1):32-39 [PDF here].
